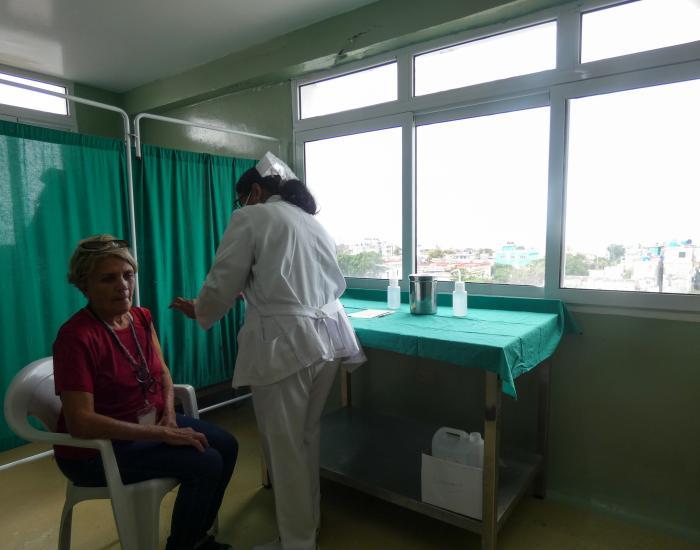


After receiving the authorization of the Center for State Control of Medicines, Equipment and Medical Devices (Cecmed) and the approval of the Ministry of Public Health, a phase II-III clinical trial began at the Institute of Hematology and Immunology, aimed at evaluating the safety, immunogenicity and efficacy of a new version of the Cuban anti-pneumococcal vaccine Quimio-Vio, in adults from 50 to 74 years of age.
Dr. Meiby de la Caridad Rodríguez González, director of Clinical Research at the Finlay Vaccine Institute (IFV), told Granma that once its safety has been proven in this age group, the trial will be extended to three health areas in the capital's Plaza de la Revolución municipality, belonging to the 19 de Abril, Abelardo Ramírez and Cosme Ordoñez polyclinics.
“The sample should include about a thousand volunteers who express their consent to participate in the study and meet the selection requirements, such as, for example, not presenting an acute infectious process at the time of vaccination, not being allergic to thiomersal or tetanus toxoid, in addition to having their underlying diseases under control.
To this end, Dr. Rodriguez Gonzalez emphasized, each person who is a candidate to participate in the trial will undergo a prior medical evaluation at the vaccination office.
“This variant of the Quimio-Vio vaccine protects against 11 of the most frequent serotypes worldwide and of greater circulation in Cuba, of the Streptococcus pneumoniae (pneumococcus) bacterium, which causes sinusitis, otitis media, pneumonias complicated with pleural effusion, as well as infections of the central nervous system and the bloodstream.
“The vaccination consists of the administration of a single dose, applied intramuscularly in the area of the arm (deltoid). In general, the expected adverse events are mild and infrequent, of a local nature, at the injection site, including pain, redness and inflammation, typical of any vaccine, and the appearance of other general effects, particularly decay, fever and headache.
With the results of this study, we intend to apply to Cecmed for the authorization of medical sanitary registration. Thus, the country would have a new vaccine for its adult population, whose application will contribute to the State's efforts to promote healthy aging, said the IFV's Director of Clinical Research.
“In Cuba, despite the fact that the greatest epidemiological burden of pneumococcal disease is concentrated in the child population under five years of age, in the adult population it also registers a high morbi-mortality.
“We have an aging population. According to data from the National Statistics and Information Office (ONEI), at the close of 2023, 24.4% of the total population was 60 years of age and older. Immunosenescence, together with the high probability of suffering from chronic diseases, make this age group very vulnerable to invasive pneumococcal disease.
Dr. Meiby de la Caridad Rodríguez stressed that, as it appears in the Health Statistical Yearbook, diseases caused by pneumococcus are the fourth cause of death in Cuba and the highest number of deaths occurs in adults over 60 years of age, mainly due to pneumonia and influenza.
He gave as an example that people aged 65 and over have a 3.8 times higher risk of suffering from pneumococcal pneumonia, compared to healthy adults aged 18 to 64. They are also 10 times more likely to be hospitalized with pneumococcal pneumonia compared to adults aged 18 to 49.
“The risk increases if they suffer from chronic diseases, such as diabetes mellitus, when this indicator increases by 2.8 times, and cardiovascular diseases, when it rises to 3.8 times, compared to healthy adults in this age group.
He recalled that last July, and after the completion of all the required clinical trials, developed over almost 13 years, the Cuban anti-pneumococcal conjugate vaccine Quimi-Vio, which protects against seven serotypes of the bacterium, received the sanitary registration granted by Cecmed, for its use in children from one to five years of age.
Since September 2024, and to date, more than 95,000 infants from two years of age have been vaccinated throughout the country, demonstrating a very good safety profile, said the researcher.
This remarkable result of Cuban science was developed by the IFV and its large-scale production takes place at the National Center for Biopreparations (Biocen).
Photo
Related News

In the spirit of the Ordinary Jubilee of 2025, the Cuban State releases persons serving se...

Yes, there are ways to continue advancing

José Martí, the one who illuminates


